Your Location:Home > Products > Vitamin & Minerals > Ubiquinol


CasNo: 992-78-9
MF: C59H92O4
Appearance: light yellow-orange solid
The invention relates to a method for sy...
This invention provides aqueous and non-...
The present invention relates to a metho...
Ubiquinol is a plasma antioxidant. The m...

ubidecarenone

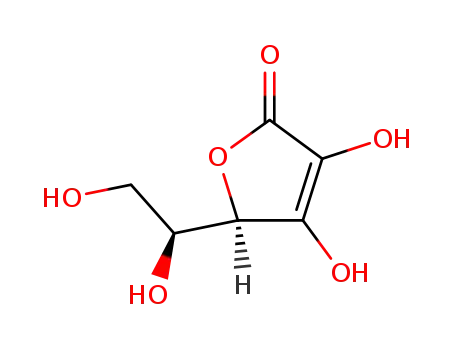
ascorbic acid


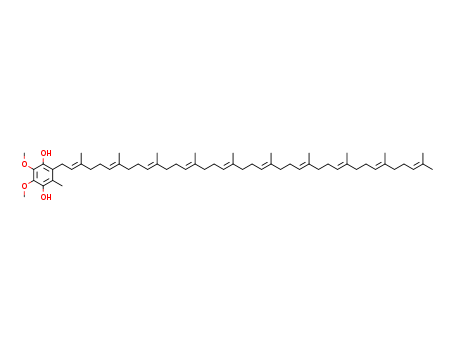
ubiquinol

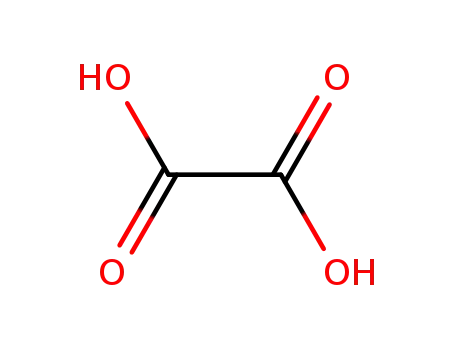
oxalic acid
| Conditions | Yield |
|---|---|
|
In ethanol; at 78 ℃; for 30h;
|

ubidecarenone


ubiquinol
| Conditions | Yield |
|---|---|
|
With sodium thiosulfate; sodium chloride; In water; at 48 ℃; for 2h; pH=4 - 6;
|
99% |
|
With sodium thiosulfate; sodium chloride; In n-heptane; water; at 25 ℃; for 2h; pH=4 - 6;
|
99% |
|
With sodium hydroxide; ascorbic acid; In ethanol; at 50 ℃;
|
98% |
|
With ascorbic acid; In ethanol; at 78 ℃; for 30h;
|
97% |
|
With sodium hydrogencarbonate; ascorbic acid; In ethanol; at 78 ℃; for 30h;
|
97% |
|
With ascorbic acid; In ethanol; at 78 ℃; for 30h; Product distribution / selectivity;
|
95% |
|
With D-limonene; water; Ascorbyl palmitate; at 80 ℃; for 16h; Concentration; Reagent/catalyst; Time; Inert atmosphere;
|
95% |
|
With sodium hydrogencarbonate; ascorbic acid; In acetone; at 50 ℃; for 45h;
|
93% |
|
With sodium dithionite; In n-heptane; water; at 25 ℃; for 2h; pH=4 - 6;
|
92.8% |
|
With acetic acid; zinc;
|
|
|
With sodium hydrogencarbonate; ascorbic acid; In ethanol; at 78 ℃; for 3h;
|
|
|
With sodium thiosulfate; In n-heptane; water; at 25 ℃; for 2h;
|
|
|
With sodium dithionite; In n-heptane; water; at 25 ℃; for 2h; pH=4 - 6; Conversion of starting material; Nitrogen atmosphere;
|
|
|
With sodium thiosulfate; In n-heptane; water; at 25 ℃; for 2h; pH=4 - 6;
|
|
|
With sodium hydrogencarbonate; ascorbic acid; In acetone; at 2 - 50 ℃; Conversion of starting material; Nitrogen atmosphere;
|
|
|
With sulfuric acid; zinc; In n-heptane; water; at 25 ℃; for 2h; pH=4 - 6; Conversion of starting material; Nitrogen atmosphere;
|
|
|
With sodium dithionite; oxygen; In hexane; water; at 25 ℃; for 2h; pH=4 - 6; Conversion of starting material;
|
|
|
With sodium dithionite; In water; ethyl acetate; at 25 ℃; for 2h; pH=4 - 6; Conversion of starting material; Nitrogen atmosphere;
|
|
|
With sodium dithionite; In n-heptane; water; at 25 ℃; for 2h; pH=4 - 6; Conversion of starting material; Nitrogen atmosphere;
|
|
|
With sodium dithionite; In water; toluene; at 25 ℃; for 2h; pH=4 - 6; Conversion of starting material; Nitrogen atmosphere;
|
|
|
With sodium dithionite; In hexane; water; at 25 ℃; for 2h; pH=4 - 6; Conversion of starting material; Nitrogen atmosphere;
|
|
|
With sodium dithionite; In hexane; water; at 25 ℃; for 2h; pH=4 - 6; Conversion of starting material; Nitrogen atmosphere;
|
|
|
With sodium dithionite; In chloroform; water; at 25 ℃; for 2h; pH=4 - 6; Conversion of starting material; Nitrogen atmosphere;
|
|
|
With sodium dithionite; In tert-butyl methyl ether; water; at 25 ℃; for 2h; pH=4 - 6; Conversion of starting material; Nitrogen atmosphere;
|
|
|
With ascorbic acid; In ethanol; water; at 2 - 78 ℃; Conversion of starting material; Nitrogen atmosphere;
|
|
|
With ascorbic acid; In ethanol; at 50 ℃; for 24h; Conversion of starting material;
|
|
|
With ascorbic acid; In ethanol; at 2 - 78 ℃; Conversion of starting material; Nitrogen atmosphere;
|
|
|
ubidecarenone; With sodium hydrogencarbonate; ascorbic acid; In acetonitrile; at 55 ℃; for 40h; Nitrogen atmosphere;
In n-heptane; water; acetonitrile; at 25 ℃; Conversion of starting material; Nitrogen atmosphere;
|
|
|
ubidecarenone; With sulfuric acid; zinc; In n-heptane; water; at 25 ℃; for 6h; Nitrogen atmosphere;
With hydrogenchloride; In n-heptane; water; Conversion of starting material; Nitrogen atmosphere;
|
|
|
ubidecarenone; With sodium dithionite; In water; ethyl acetate; at 25 ℃; for 2h; pH=4 - 6; Nitrogen atmosphere;
In ethanol; water; ethyl acetate; at 2 ℃; Conversion of starting material; Nitrogen atmosphere;
|
|
|
ubidecarenone; With sodium dithionite; In water; ethyl acetate; at 25 ℃; for 2h; pH=4 - 6; Nitrogen atmosphere;
In ethanol; water; ethyl acetate; at 2 - 48 ℃; Conversion of starting material; Nitrogen atmosphere;
|
|
|
ubidecarenone; With sodium dithionite; In hexane; water; at 25 ℃; for 2h; pH=4 - 6; Nitrogen atmosphere;
In methanol; hexane; at 2 ℃; Conversion of starting material; Nitrogen atmosphere;
|
|
|
ubidecarenone; With sodium dithionite; In hexane; water; at 25 ℃; for 2h; pH=4 - 6; Nitrogen atmosphere;
In ethanol; at 2 - 50 ℃; Conversion of starting material; Nitrogen atmosphere;
|
|
|
ubidecarenone; With sodium dithionite; In water; at 50 ℃; for 2h; pH=4 - 6; Nitrogen atmosphere;
In ethanol; at 2 - 50 ℃; Conversion of starting material; Nitrogen atmosphere;
|
|
|
ubidecarenone; With sodium dithionite; In water; at 2 - 50 ℃; pH=4 - 6; Nitrogen atmosphere;
at 2 ℃; Conversion of starting material;
|
|
|
ubidecarenone; With sulfuric acid; zinc; In n-heptane; water; at 25 ℃; for 6h; Nitrogen atmosphere;
With hydrogenchloride; In n-heptane; water; Nitrogen atmosphere;
In ethanol; at 2 - 50 ℃; Conversion of starting material; Nitrogen atmosphere;
|
|
|
With sodium dithionite; In n-heptane; water; at 25 ℃; for 2h; pH=4 - 6; Product distribution / selectivity;
|
|
|
With ascorbic acid; In ethanol; at 78 ℃; for 30h;
|
|
|
With sodium dithionite; In n-heptane; water; at 25 ℃; for 2h; pH=4 - 6;
|
|
|
With ascorbic acid; In ethanol; at 78 ℃; for 30h;
|
|
|
With acetic acid; zinc; at 65 - 70 ℃; for 1h; Heating / reflux;
|
|
|
With ascorbic acid; In ethanol; at 78 ℃; for 30h; Product distribution / selectivity;
|
|
|
With sodium thiosulfate; In n-heptane; water; at 25 ℃; for 2h; pH=4 - 6; Product distribution / selectivity;
|
|
|
With sodium dithionite; In water; for 2h; Purification / work up;
|
|
|
With dihydrolipoic acid; at 4 - 50 ℃; for 30 - 1680h; pH=3.8 - 3.9; Product distribution / selectivity;
|
|
|
With sodium tetrahydroborate; In tetrahydrofuran; at 30 ℃; for 1h; Product distribution / selectivity;
|
|
|
With ascorbic acid; In ethanol; water; at 78 ℃; pH=3.6; Product distribution / selectivity;
|
|
|
With sodium dithionite; In hexane; Inert atmosphere;
|
|
|
With zinc pheophorbide a; ascorbic acid; Reagent/catalyst; Catalytic behavior; Irradiation; Inert atmosphere;
|
|
|
With Vitamin C; In water; at 90 - 95 ℃; for 1h; Solvent; Reagent/catalyst; Large scale;
|
|
|
With D-ascorbic acid; In ethanol; at 78 ℃; for 30h;
|
|
|
With sodium thiosulfate; In hexane; at 20 ℃; for 2h;
|
|
|
With sodium dithionite; In n-heptane; water; at 25 ℃; for 2h; pH=4 - 6;
|
|
|
With ascorbic acid; In ethanol; at 78 ℃; for 30h;
|
|
|
With ascorbic acid; In ethanol; at 78 ℃; for 30h;
|

1,4-Bis-benzyloxy-2-((2E,6E,10E,14E,18E,22E,26E,30E,34E)-3,7,11,15,19,23,27,31,35,39-decamethyl-tetraconta-2,6,10,14,18,22,26,30,34,38-decaenyl)-5,6-dimethoxy-3-methyl-benzene
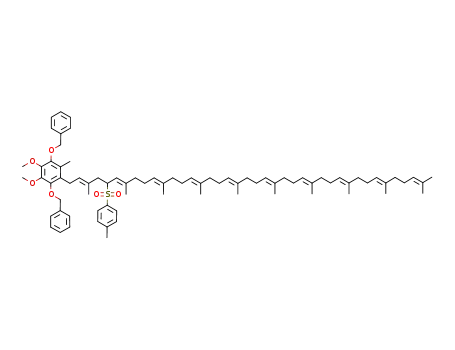
1,4-bis(benzyloxy)-2-{(2E,6E,10E,14E,18E,22E,26E,30E,34E)-3,7,11,15,19,23,27,31,35,39-decamethyl-5-[(4-methylphenyl)sulfonyl]tetraconta-2,6,10,14,18,22,26,30,34,38-decaenyl}-5,6-dimethoxy-3-methylbenzene

1-((2E,6E,10E,14E,18E,22E,26E,30E,34E)-3,7,11,15,19,23,27,31,35,39-decamethyltetraconta-2,6,10,14,18,22,26,30,34,38-decaenyl)-3,4-dimethoxy-2,5-bis((2-methoxyethoxy)methoxy)-6-methylbenzene

ubidecarenone

ubidecarenone
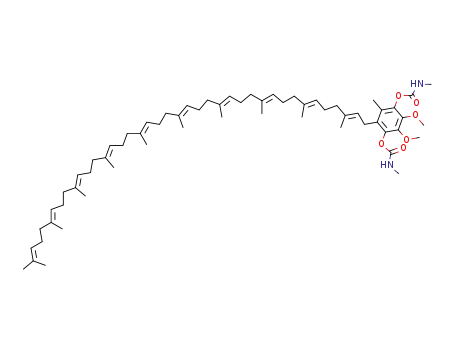
Hydronbichinon-bis-methylcarbamat
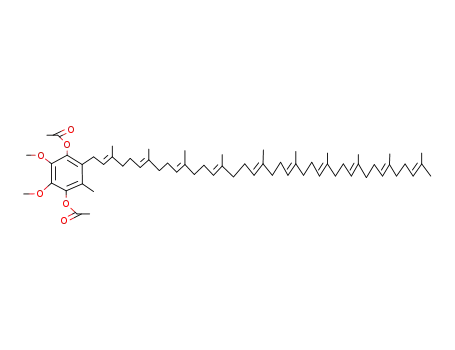
1,4-diacetoxy-2,3-dimethoxy-5-methyl-6-decaprenylbenzene
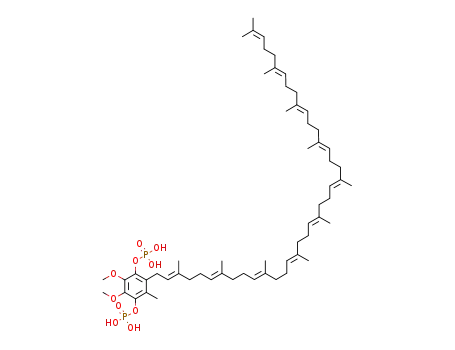
C59H94O10P2