Your Location:Home > Products > Anti-aging > Sulfuretin
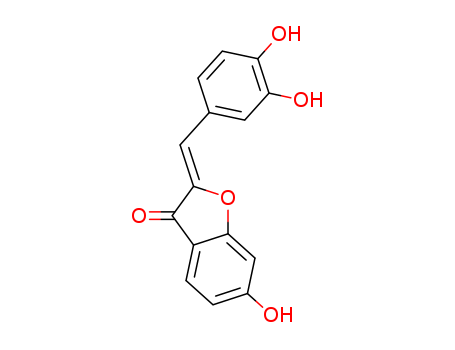


CasNo: 120-05-8
MF: C15H10 O5
|
General Description |
Sulfuretin is a natural organic compound that belongs to the flavonoid group of chemicals. It is found in various plants, including Sophora japonica and Rhus succedanea, and is responsible for the yellow and orange pigmentation in some flowers and fruits. Sulfuretin has been studied for its potential medicinal and therapeutic properties, including antioxidant, anti-inflammatory, and anti-cancer effects. It has also shown promising results in the treatment of skin conditions such as dermatitis and eczema. Additionally, sulfuretin has been investigated for its role in aiding insulin sensitivity and reducing the risk of diabetes. Research on sulfuretin is ongoing to further explore its potential applications in medicine and healthcare. |
InChI:InChI=1/C15H10O5/c16-9-2-3-10-13(7-9)20-14(15(10)19)6-8-1-4-11(17)12(18)5-8/h1-7,16-18H/b14-6+
To widen aurones applicability in achrom...
In this work, we describe the design, sy...
Background: Aurones, (Z)-2-benzylidenebe...
A simple and green method for the synthe...
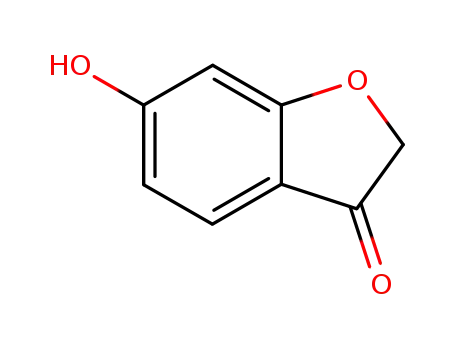
6-hydroxybenzofuran-3-one

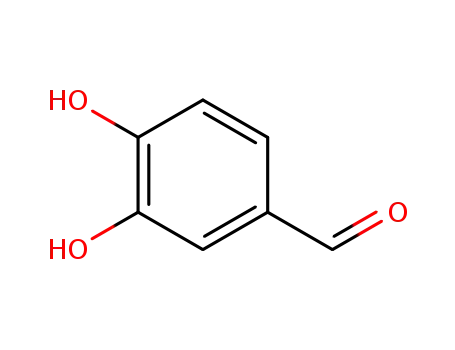
3,4-dihydroxybenzaldehyde

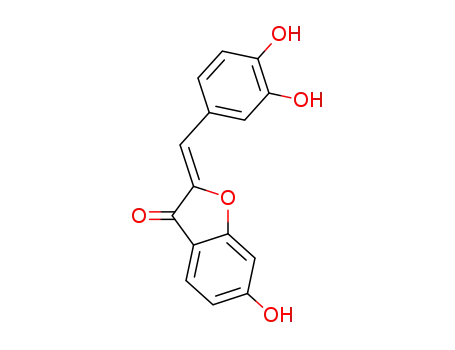
sulfuretin
| Conditions | Yield |
|---|---|
|
With potassium hydroxide; In ethanol; water; for 3h; Reflux;
|
85% |
|
In water; for 10h; Reflux; Green chemistry;
|
81% |
|
In water; Reflux; Green chemistry;
|
81% |
|
With hydrogenchloride; In ethanol; water; Reflux;
|
60% |
|
With potassium hydroxide; In ethanol; water; at 80 ℃;
|
26% |
|
With hydrogenchloride; In water; isopropyl alcohol; at 80 ℃; for 16h;
|
5% |
|
With hydrogenchloride;
|
|
|
With hydrogenchloride; acetic acid;
|
|
|
With hydrogenchloride; ethanol;
|
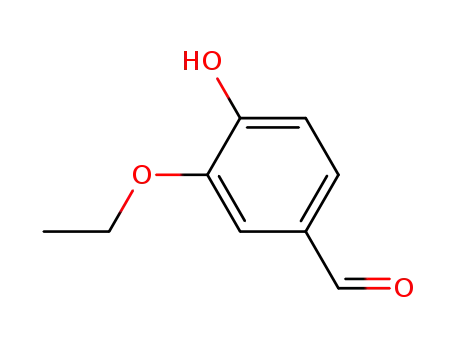
4-hydroxy-3-ethoxybenzaldehyde


6-hydroxybenzofuran-3-one


sulfuretin
| Conditions | Yield |
|---|---|
|
With potassium hydroxide; In methanol; water; for 3h; Reflux; Inert atmosphere;
|
44% |

6-hydroxybenzofuran-3-one

3,4-dihydroxybenzaldehyde
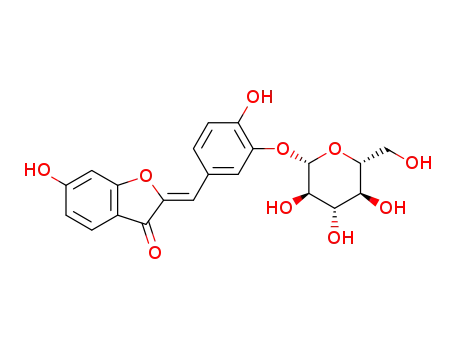
sulphuretin 3'-O-β-glucopyranoside
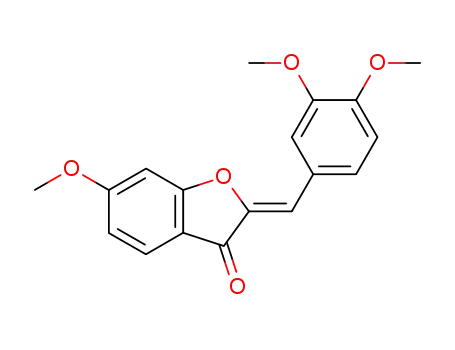
(Z)-2-(3,4-dimethoxybenzylidene)-6-methoxybenzofuran-3(2H)-one
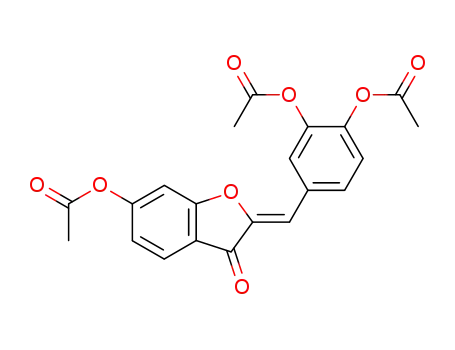
6-acetoxy-2-((Z)-3,4-diacetoxy-benzylidene)-benzofuran-3-one
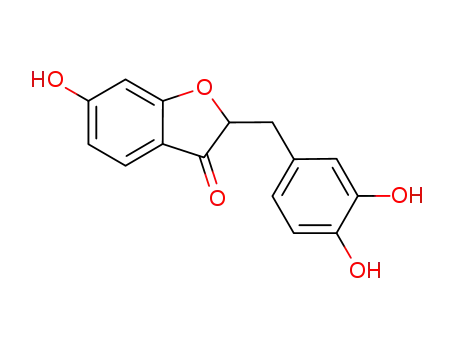
2-(3,4-dihydroxybenzyl)-6-hydroxybenzofuran-3(2H)-one