Your Location:Home > Products > Anti-aging > Fisetine
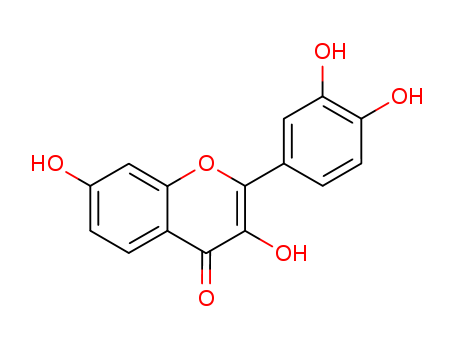


CasNo: 528-48-3
MF: C15H10O6
Appearance: yellow to brown crystalline powder
|
Anticancer Research |
Fisetin is a plant polyphenol from the flavonoid group. It occurs in fruits and vegetablesincluding persimmons, strawberries, onions, cucumbers, and apples. It is anantioxidant, exerts anticarcinogenic effects in HCT-116 (human colon carcinoma)cells, and modulates protein kinase and lipid kinase pathways (Wang et al. 2012).Fisetin alter signaling pathway like MAPK, NF-κB, activators of transcription(JAK/STAT), Janus kinase/signal transducers, phosphoinositide-3-kinase-proteinkinase (PI3K/Akt), Wnt, and mammalian target of rapamycin (mTOR), therebyleading to cell cycle arrest in HL-60 cells (human acute promyelocytic leukemiacells) (Singh et al. 2016b). Thus, it exhibits inhibitory effects on adhesion, migration,invasion, and multidrug resistance (Suh et al. 2009). |
|
General Description |
This substance is a primary reference substance with assigned absolute purity (considering chromatographic purity, water, residual solvents, inorganic impurities). The exact value can be found on the certificate. Produced by PhytoLab GmbH & Co. KG |
InChI:InChI=1/C15H10O6/c16-8-2-3-9-12(6-8)21-15(14(20)13(9)19)7-1-4-10(17)11(18)5-7/h1-6,16-18,20H
The invention provides a method for synt...
Fisetin and 2′,4′,6′-trihydroxydihyrocha...
Flavonoids represent a potential source ...
The invention provides a genetically mod...
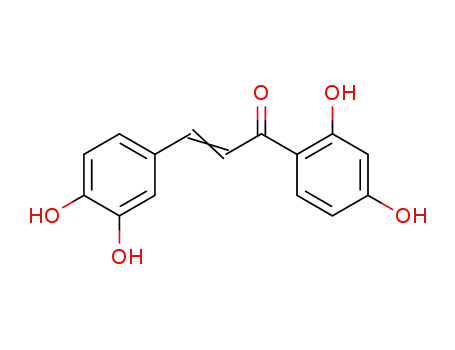
3,4,2',4'-tetrahydroxy-chalcone

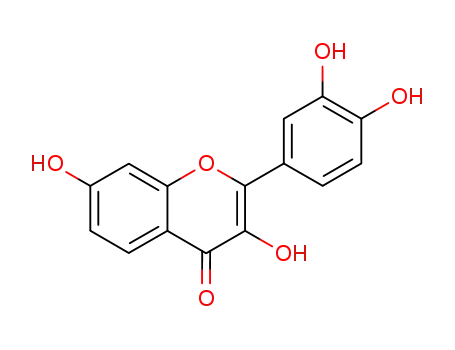
3,7,3',4'-tetrahydroxyflavone
| Conditions | Yield |
|---|---|
|
With sodium carbonate / sodium bicarbonate buffer solution; potassium hydrogen sulfate complex; In dichloromethane; acetone; at 20 ℃; for 21h;
|
96.7% |
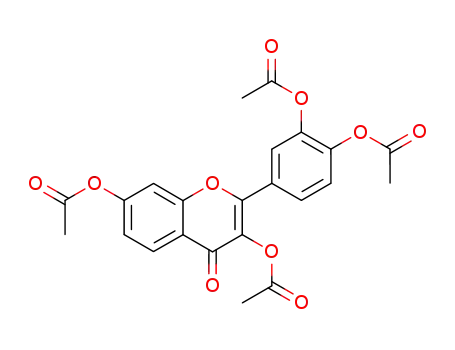
3,3′,4′,7-tetraacetoxyflavone


3,7,3',4'-tetrahydroxyflavone
| Conditions | Yield |
|---|---|
|
With sodium dithionite; water; sodium hydroxide; In methanol; at 20 ℃; for 4h; Inert atmosphere;
|
65% |
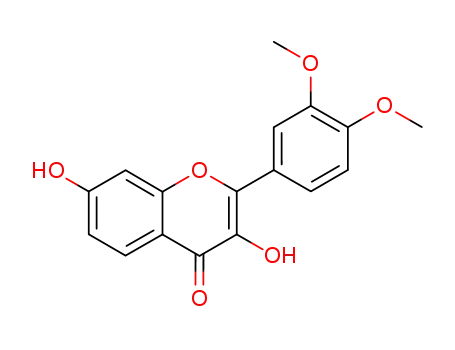
Fisetin 3',4'-dimethyl ether
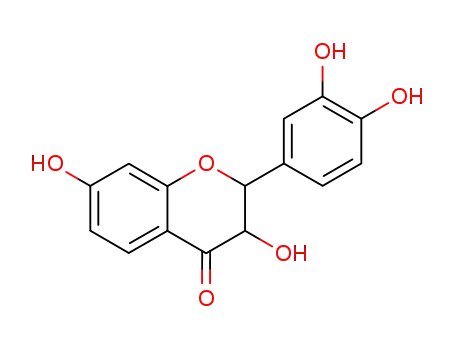
3,7,3',4'-tetrahydroxyflavanone
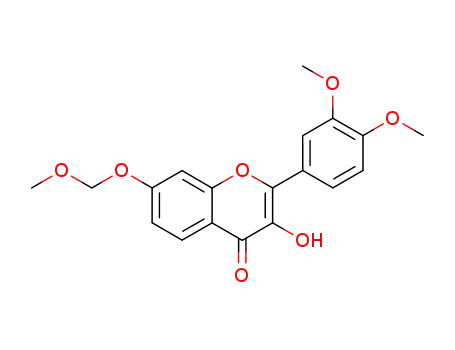
2-(3,4-dimethoxy-phenyl)-3-hydroxy-7-methoxymethoxy-chromen-4-one
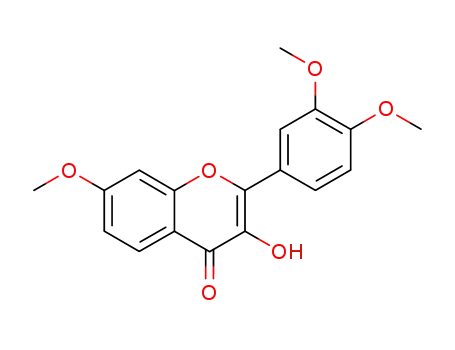
2-(3,4-dimethoxyphenyl)-3-hydroxy-7-methoxy-4H-chromen-4-one
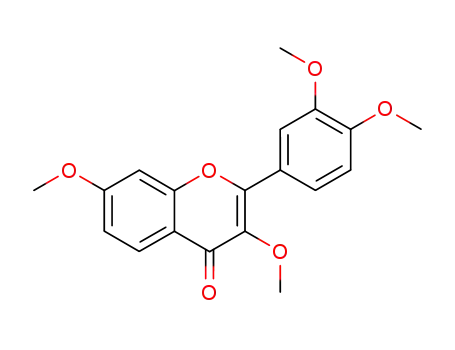
3,3',4',7-tetra-O-methylfisetin

3,3′,4′,7-tetraacetoxyflavone
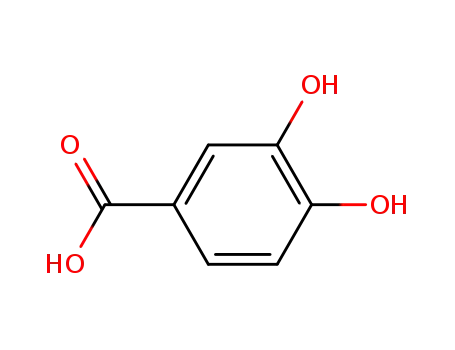
3,4-Dihydroxybenzoic acid
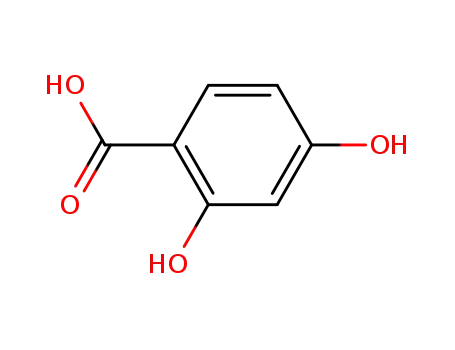
4-hydroxysalicylic acid