Your Location:Home > Products > Vitamin & Minerals > Ubiquinol
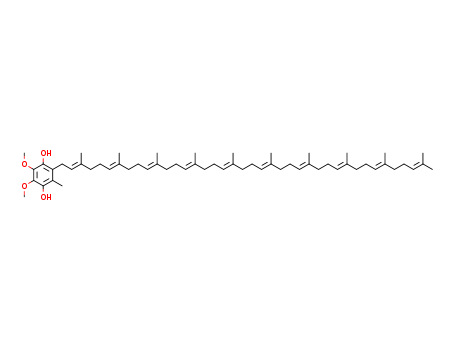


CasNo: 992-78-9
MF: C59H92O4
Appearance: light yellow-orange solid
Quality products make an important contribution to long-term revenue and profitability. Cost-effective customized wholesale Ubiquinol 992-78-9
Reduced coenzyme Q for improving nervous system cell functions

ubidecarenone

ascorbic acid


ubiquinol

oxalic acid
| Conditions | Yield |
|---|---|
|
In
ethanol;
at 78 ℃;
for 30h;
|

ubidecarenone


ubiquinol
| Conditions | Yield |
|---|---|
|
With
sodium thiosulfate; sodium chloride;
In
water;
at 48 ℃;
for 2h;
pH=4 - 6;
|
99% |
|
With
sodium thiosulfate; sodium chloride;
In
n-heptane; water;
at 25 ℃;
for 2h;
pH=4 - 6;
|
99% |
|
With
sodium hydroxide; ascorbic acid;
In
ethanol;
at 50 ℃;
|
98% |
|
With
ascorbic acid;
In
ethanol;
at 78 ℃;
for 30h;
|
97% |
|
With
sodium hydrogencarbonate; ascorbic acid;
In
ethanol;
at 78 ℃;
for 30h;
|
97% |
|
With
ascorbic acid;
In
ethanol;
at 78 ℃;
for 30h;
Product distribution / selectivity;
|
95% |
|
With
D-limonene; water; Ascorbyl palmitate;
at 80 ℃;
for 16h;
Concentration;
Reagent/catalyst;
Time;
Inert atmosphere;
|
95% |
|
With
sodium hydrogencarbonate; ascorbic acid;
In
acetone;
at 50 ℃;
for 45h;
|
93% |
|
With
sodium dithionite;
In
n-heptane; water;
at 25 ℃;
for 2h;
pH=4 - 6;
|
92.8% |
|
With
acetic acid; zinc;
|
|
|
With
sodium hydrogencarbonate; ascorbic acid;
In
ethanol;
at 78 ℃;
for 3h;
|
|
|
With
sodium thiosulfate;
In
n-heptane; water;
at 25 ℃;
for 2h;
|
|
|
With
sodium dithionite;
In
n-heptane; water;
at 25 ℃;
for 2h;
pH=4 - 6;
Conversion of starting material;
Nitrogen atmosphere;
|
|
|
With
sodium thiosulfate;
In
n-heptane; water;
at 25 ℃;
for 2h;
pH=4 - 6;
|
|
|
With
sodium hydrogencarbonate; ascorbic acid;
In
acetone;
at 2 - 50 ℃;
Conversion of starting material;
Nitrogen atmosphere;
|
|
|
With
sulfuric acid; zinc;
In
n-heptane; water;
at 25 ℃;
for 2h;
pH=4 - 6;
Conversion of starting material;
Nitrogen atmosphere;
|
|
|
With
sodium dithionite; oxygen;
In
hexane; water;
at 25 ℃;
for 2h;
pH=4 - 6;
Conversion of starting material;
|
|
|
With
sodium dithionite;
In
water; ethyl acetate;
at 25 ℃;
for 2h;
pH=4 - 6;
Conversion of starting material;
Nitrogen atmosphere;
|
|
|
With
sodium dithionite;
In
n-heptane; water;
at 25 ℃;
for 2h;
pH=4 - 6;
Conversion of starting material;
Nitrogen atmosphere;
|
|
|
With
sodium dithionite;
In
water; toluene;
at 25 ℃;
for 2h;
pH=4 - 6;
Conversion of starting material;
Nitrogen atmosphere;
|
|
|
With
sodium dithionite;
In
hexane; water;
at 25 ℃;
for 2h;
pH=4 - 6;
Conversion of starting material;
Nitrogen atmosphere;
|
|
|
With
sodium dithionite;
In
hexane; water;
at 25 ℃;
for 2h;
pH=4 - 6;
Conversion of starting material;
Nitrogen atmosphere;
|
|
|
With
sodium dithionite;
In
chloroform; water;
at 25 ℃;
for 2h;
pH=4 - 6;
Conversion of starting material;
Nitrogen atmosphere;
|
|
|
With
sodium dithionite;
In
tert-butyl methyl ether; water;
at 25 ℃;
for 2h;
pH=4 - 6;
Conversion of starting material;
Nitrogen atmosphere;
|
|
|
With
ascorbic acid;
In
ethanol; water;
at 2 - 78 ℃;
Conversion of starting material;
Nitrogen atmosphere;
|
|
|
With
ascorbic acid;
In
ethanol;
at 50 ℃;
for 24h;
Conversion of starting material;
|
|
|
With
ascorbic acid;
In
ethanol;
at 2 - 78 ℃;
Conversion of starting material;
Nitrogen atmosphere;
|
|
|
ubidecarenone;
With
sodium hydrogencarbonate; ascorbic acid;
In
acetonitrile;
at 55 ℃;
for 40h;
Nitrogen atmosphere;
In
n-heptane; water; acetonitrile;
at 25 ℃;
Conversion of starting material;
Nitrogen atmosphere;
|
|
|
ubidecarenone;
With
sulfuric acid; zinc;
In
n-heptane; water;
at 25 ℃;
for 6h;
Nitrogen atmosphere;
With
hydrogenchloride;
In
n-heptane; water;
Conversion of starting material;
Nitrogen atmosphere;
|
|
|
ubidecarenone;
With
sodium dithionite;
In
water; ethyl acetate;
at 25 ℃;
for 2h;
pH=4 - 6;
Nitrogen atmosphere;
In
ethanol; water; ethyl acetate;
at 2 ℃;
Conversion of starting material;
Nitrogen atmosphere;
|
|
|
ubidecarenone;
With
sodium dithionite;
In
water; ethyl acetate;
at 25 ℃;
for 2h;
pH=4 - 6;
Nitrogen atmosphere;
In
ethanol; water; ethyl acetate;
at 2 - 48 ℃;
Conversion of starting material;
Nitrogen atmosphere;
|
|
|
ubidecarenone;
With
sodium dithionite;
In
hexane; water;
at 25 ℃;
for 2h;
pH=4 - 6;
Nitrogen atmosphere;
In
methanol; hexane;
at 2 ℃;
Conversion of starting material;
Nitrogen atmosphere;
|
|
|
ubidecarenone;
With
sodium dithionite;
In
hexane; water;
at 25 ℃;
for 2h;
pH=4 - 6;
Nitrogen atmosphere;
In
ethanol;
at 2 - 50 ℃;
Conversion of starting material;
Nitrogen atmosphere;
|
|
|
ubidecarenone;
With
sodium dithionite;
In
water;
at 50 ℃;
for 2h;
pH=4 - 6;
Nitrogen atmosphere;
In
ethanol;
at 2 - 50 ℃;
Conversion of starting material;
Nitrogen atmosphere;
|
|
|
ubidecarenone;
With
sodium dithionite;
In
water;
at 2 - 50 ℃;
pH=4 - 6;
Nitrogen atmosphere;
at 2 ℃;
Conversion of starting material;
|
|
|
ubidecarenone;
With
sulfuric acid; zinc;
In
n-heptane; water;
at 25 ℃;
for 6h;
Nitrogen atmosphere;
With
hydrogenchloride;
In
n-heptane; water;
Nitrogen atmosphere;
In
ethanol;
at 2 - 50 ℃;
Conversion of starting material;
Nitrogen atmosphere;
|
|
|
With
sodium dithionite;
In
n-heptane; water;
at 25 ℃;
for 2h;
pH=4 - 6;
Product distribution / selectivity;
|
|
|
With
ascorbic acid;
In
ethanol;
at 78 ℃;
for 30h;
|
|
|
With
sodium dithionite;
In
n-heptane; water;
at 25 ℃;
for 2h;
pH=4 - 6;
|
|
|
With
ascorbic acid;
In
ethanol;
at 78 ℃;
for 30h;
|
|
|
With
acetic acid; zinc;
at 65 - 70 ℃;
for 1h;
Heating / reflux;
|
|
|
With
ascorbic acid;
In
ethanol;
at 78 ℃;
for 30h;
Product distribution / selectivity;
|
|
|
With
sodium thiosulfate;
In
n-heptane; water;
at 25 ℃;
for 2h;
pH=4 - 6;
Product distribution / selectivity;
|
|
|
With
sodium dithionite;
In
water;
for 2h;
Purification / work up;
|
|
|
With
dihydrolipoic acid;
at 4 - 50 ℃;
for 30 - 1680h;
pH=3.8 - 3.9;
Product distribution / selectivity;
|
|
|
With
sodium tetrahydroborate;
In
tetrahydrofuran;
at 30 ℃;
for 1h;
Product distribution / selectivity;
|
|
|
With
ascorbic acid;
In
ethanol; water;
at 78 ℃;
pH=3.6;
Product distribution / selectivity;
|
|
|
With
sodium dithionite;
In
hexane;
Inert atmosphere;
|
|
|
With
zinc pheophorbide a; ascorbic acid;
Reagent/catalyst;
Catalytic behavior;
Irradiation;
Inert atmosphere;
|
|
|
With
Vitamin C;
In
water;
at 90 - 95 ℃;
for 1h;
Solvent;
Reagent/catalyst;
Large scale;
|
|
|
With
D-ascorbic acid;
In
ethanol;
at 78 ℃;
for 30h;
|
|
|
With
sodium thiosulfate;
In
hexane;
at 20 ℃;
for 2h;
|
|
|
With
sodium dithionite;
In
n-heptane; water;
at 25 ℃;
for 2h;
pH=4 - 6;
|
|
|
With
ascorbic acid;
In
ethanol;
at 78 ℃;
for 30h;
|
|
|
With
ascorbic acid;
In
ethanol;
at 78 ℃;
for 30h;
|
The CAS number of UBIQUINOL is 992-78-9.
More information of UBIQUINOL 992-78-9 are:
|
CAS Number |
992-78-9 |
|
Density |
0.951±0.06 g/cm3(Predicted) |
|
Melting Point |
48-49 °C |
|
Boiling Point |
866.9±65.0 °C(Predicted) |
|
HS CODE |
2909500000 |
|
PSA |
58.92000 |
|
LogP |
18.47060 |
|
Pka |
10.53±0.50(Predicted) |
Synonyms for UBIQUINOL 992-78-9:1,4-Benzenediol,2-(3,7,11,15,19,23,27,31,35,39-decamethyl-2,6,10,14,18,22,26,30,34,38-tetracontadecaenyl)-5,6-dimethoxy-3-methyl-,(all-E)-;Hydroquinone,2-(3,7,11,15,19,23,27,31,35,39-decamethyl-2,6,10,14,18,22,26,30,34,38-tetracontadecaenyl)-5,6-dimethoxy-3-methyl-(6CI,7CI,8CI);Coenzyme Q10, dihydro-;Dihydrocoenzyme Q10;Kaneka QH;Reduced coenzyme Q10;Reduced ubiquinone Q10;Ubiquinol 50;
The chemical formula of UBIQUINOL is C59H92O4 which containing 59 Carbon atoms,92 Hydrogen atoms and 4 Oxygen atoms,and the molecular weight of UBIQUINOL is 865.377.
Ubiquinol is a reduced form of coenzyme Q10 (CoQ10; ), which exists in three redox states: fully oxidized (CoQ10/ubiquinone), partially reduced (semiquinone/ubisemiquinone), and fully reduced (ubiquinol). CoQ10 acts as an electron shuttle in the electron transport chain via its reduction to ubiquinol between mitochondrial complexes I and II, also known as NADH dehydrogenase and succinate dehydrogenase, respectively, and mitochondrial complex III, also known as cytochrome bc1 complex. CoQ10 is also reduced to ubiquinol by ferroptosis suppressor protein 1 (FSP1) with NADPH as a cofactor, and ubiquinol traps lipid peroxyl radicals and inhibits lipid peroxidation helping to prevent ferroptosis.
Relevant articles related to UBIQUINOL:
|
Article |
Source |
|
FORMULATIONS OF LIPOPHILIC BIOACTIVE MOLECULES |
- Paragraph 0365; 0366; 0367, (2014/02/16) |
|
Dietary chlorophyll metabolites catalyze the photoreduction of plasma ubiquinone |
Qu, Jinfeng,Ma, Li,Zhang, Junhua,Jockusch, Steffen,Washington, Ilyas , p. 310 - 313 (2013/07/19) |
ANTAI BIO-TECH CO LTD is a quality supplier of UBIQUINOL. Our main goal is customer satisfaction. Contact us to negotiate the best price for your business on UBIQUINOL 992-78-9.