Your Location:Home > Products > Nutritional Supplement > Phenibut (β-Phenyl-GABA HCl)
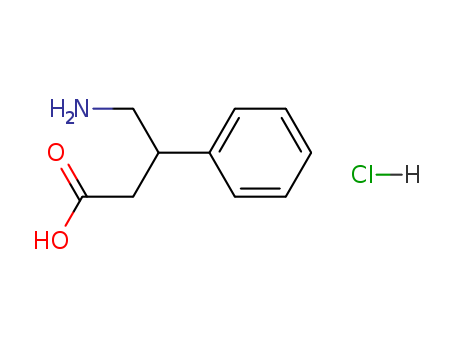


CasNo: 3060-41-1
MF: C10H14ClNO2
Appearance: White crystalline powder
InChI:InChI=1/C10H13NO2.ClH/c11-9(7-10(12)13)6-8-4-2-1-3-5-8;/h1-5,9H,6-7,11H2,(H,12,13);1H
The first divergent synthesis of both γ-...
The invention discloses a preparation me...
In this paper, we report the assembling ...
Pregabalin,baclofen,and3-phenibutareγ-am...
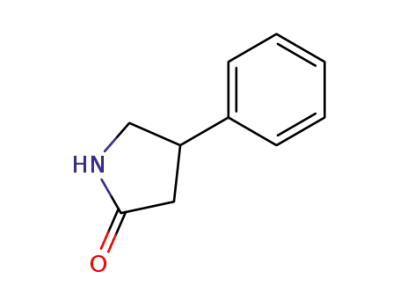
4-phenylpyrrolidin-2-one

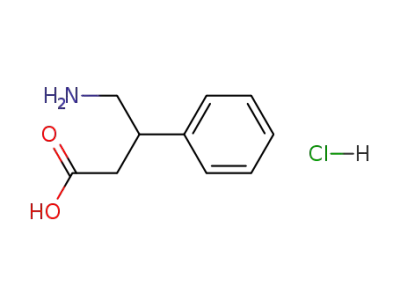
(±)-3-carboxy-2-phenylpropan-1-aminium chloride
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride; water;
for 12h;
Reflux;
|
88% |
|
With
hydrogenchloride;
In
water;
for 12h;
Reflux;
|
88% |
|
With
hydrogenchloride;
In
water;
for 16h;
Sealed tube;
Reflux;
|
65% |
|
With
hydrogenchloride; water;
In
water;
for 16h;
Reflux;
|
55% |
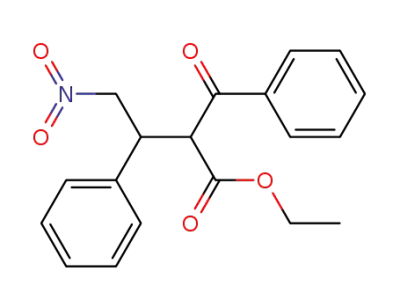
α-phenyl-β-benzoyl-γ-nitrobutyrate ethyl ester


(±)-3-carboxy-2-phenylpropan-1-aminium chloride
| Conditions | Yield |
|---|---|
|
α-phenyl-β-benzoyl-γ-nitrobutyrate ethyl ester;
With
hydrogen;
In
methanol;
at 38 - 42 ℃;
for 7h;
under 11251.1 - 30003 Torr;
With
hydrogenchloride;
In
water;
at 90 - 100 ℃;
for 1h;
Reagent/catalyst;
|
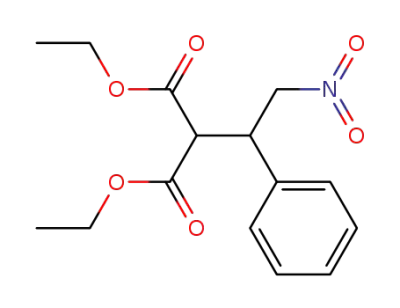
diethyl 2-(3-nitrophenylethyl)malonate

4-phenylpyrrolidin-2-one
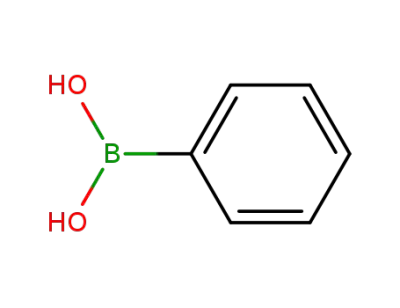
phenylboronic acid
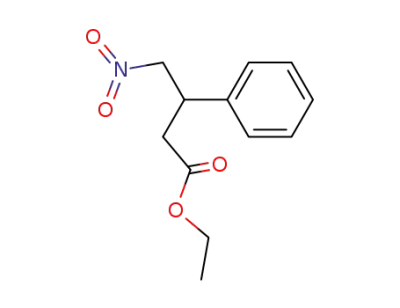
ethyl 3-phenyl-4-nitrobutanoate
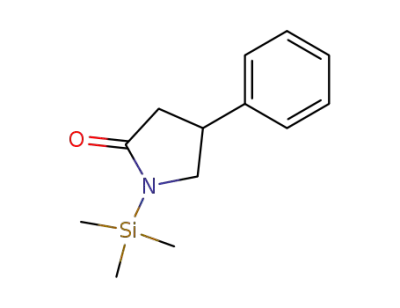
4-phenyl-1-(trimethylsilyl)-2-pyrrolidinone
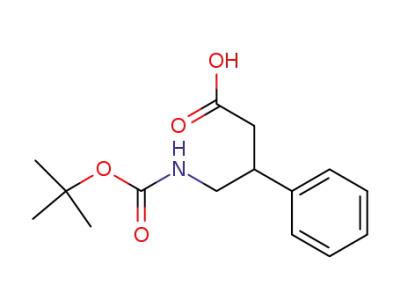
4-((tert-butoxycarbonyl)amino)-3-phenylbutanoic acid
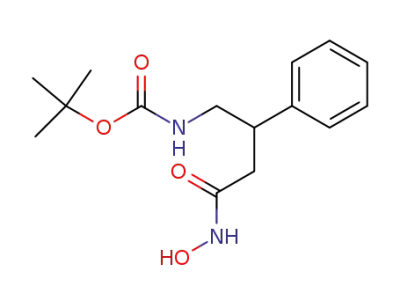
(3-Hydroxycarbamoyl-2-phenyl-propyl)-carbamic acid tert-butyl ester
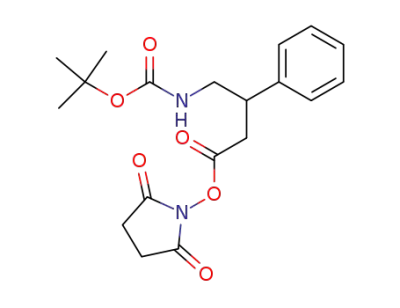
4-tert-Butoxycarbonylamino-3-phenyl-butyric acid 2,5-dioxo-pyrrolidin-1-yl ester