Your Location:Home > Products > Amino Acids > BHB (Mg/Ca/Na salts have no single CAS)
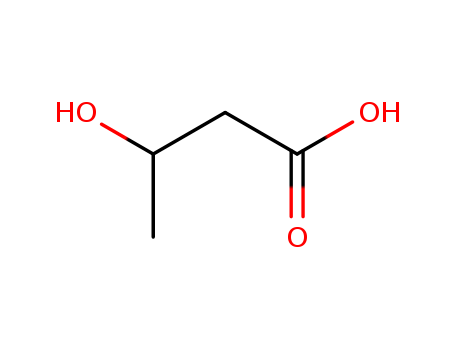


CasNo: 300-85-6
MF: C4H8O3
|
Definition |
ChEBI: A straight-chain 3-hydroxy monocarboxylic acid comprising a butyric acid core with a single hydroxy substituent in the 3- position; a ketone body whose levels are raised during ketosis, used as an energy source by the brain during fasting in humans. Also u ed to synthesise biodegradable plastics. |
InChI:InChI=1/C4H8O3/c1-3(5)2-4(6)7/h3,5H,2H2,1H3,(H,6,7)/p-1/t3-/m1/s1
Nitrogen-doped cobalt nanoparticles load...
We report a reaction platform for the sy...
The practical application of Shilov-type...
The invention discloses a method for pre...
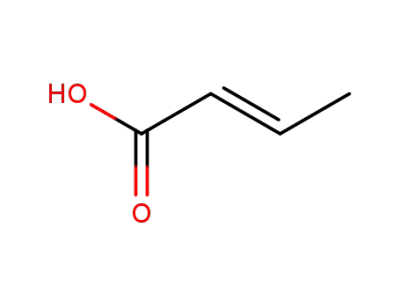
(E)-but-2-enoic acid

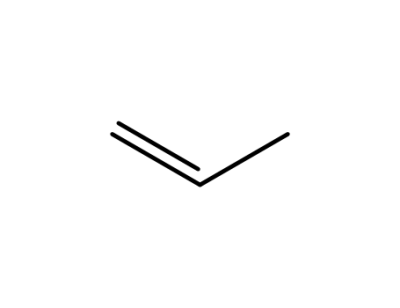
propene

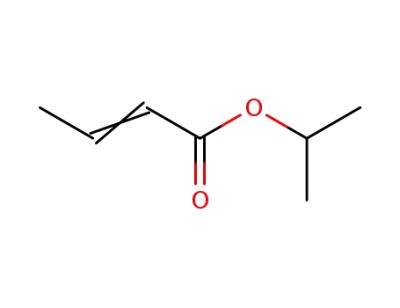
crotonic acid isopropyl ester

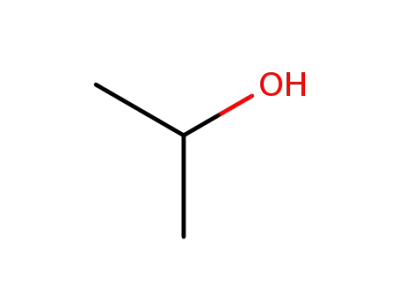
isopropyl alcohol

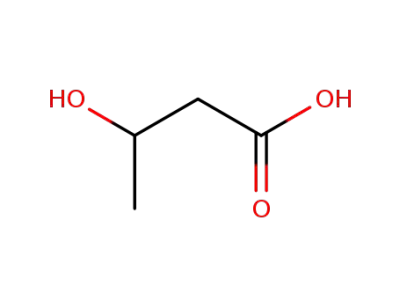
3-Hydroxybutyric acid
| Conditions | Yield |
|---|---|
|
With
water;
at 230 ℃;
for 1h;
Product distribution;
1M Solution of acid.;
|
4 % Chromat. 2 % Chromat. 3.3 % Chromat. |
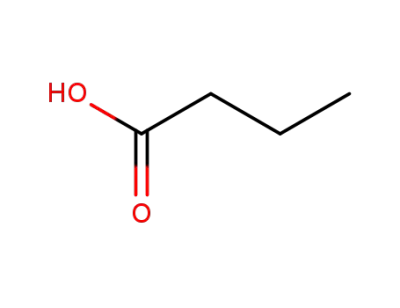
butyric acid

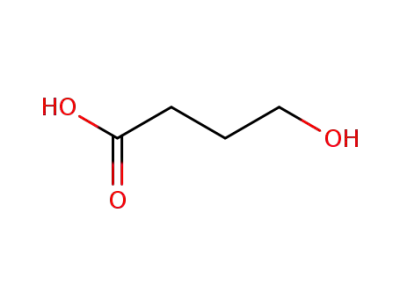
4-hydroxybutanoic acid


3-Hydroxybutyric acid
| Conditions | Yield |
|---|---|
|
With
carbon monoxide; oxygen; trifluoroacetic acid;
palladium on activated charcoal; copper dichloride;
at 75 ℃;
for 18h;
under 56887.8 Torr;
Title compound not separated from byproducts.;
|
24 % Spectr. 22% |
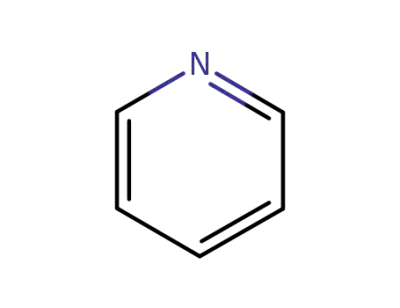
pyridine
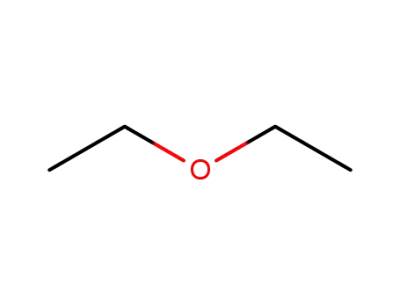
diethyl ether
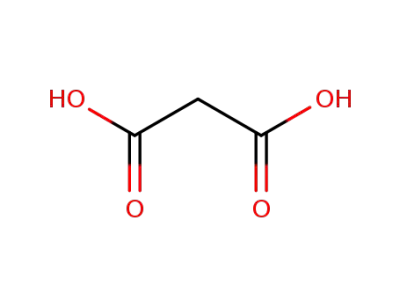
malonic acid
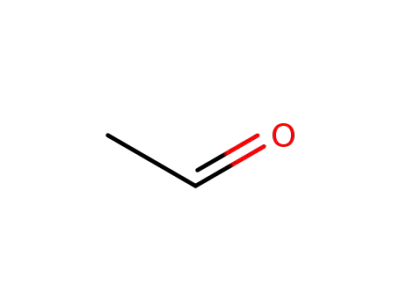
acetaldehyde
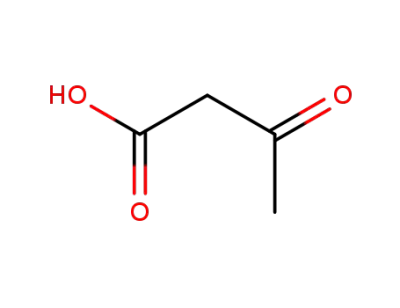
2-acetoacetic acid
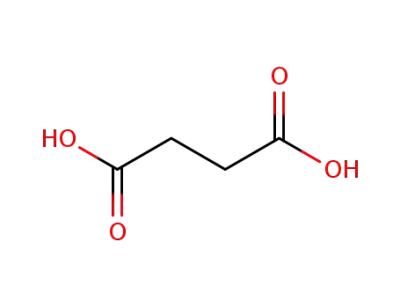
succinic acid
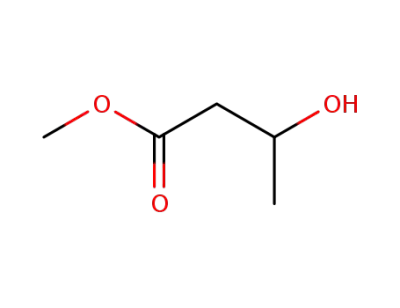
3-hydroxybutyric acid methyl ester
citric acid