Your Location:Home > Products > ANTI-AGING > Phytosphingosine
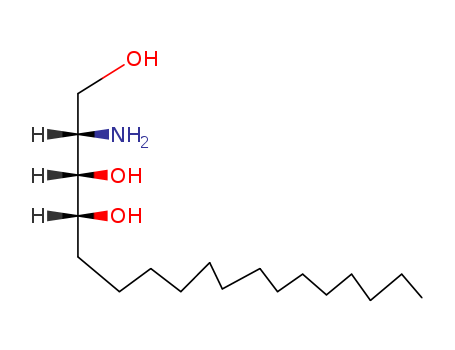


CasNo: 554-62-1
MF: C18H39NO3
|
Flammability and Explosibility |
Notclassified |
|
Veterinary Drugs and Treatments |
Phytosphingosine is a unique topical antiseborrheic compound. It is in a class called ceramides which are waxy materials meant to meant to mimic the normal lipid composition of the stratum corneum. It may also have some antiinflammatory and antimicrobial properties. |
InChI:InChI=1/C18H39NO3/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-17(21)18(22)16(19)15-20/h16-18,20-22H,2-15,19H2,1H3/t16-,17+,18-/m0/s1
-
A new ceramide, named hygrophamide (1), ...
Phytochemical investigation of seed coat...
The new phytosphingosine-type ceramide 1...
The bacterial domain produces numerous t...
An eco-friendly dual catalyst system for...
Divergent total synthesis of D-ribo-phyt...
N-Boc-α-amino aldehydes are shown to be ...
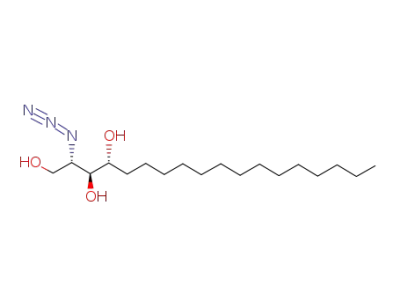
(2S,3S,4R)-2-azido-1,3,4-octadecanetriol

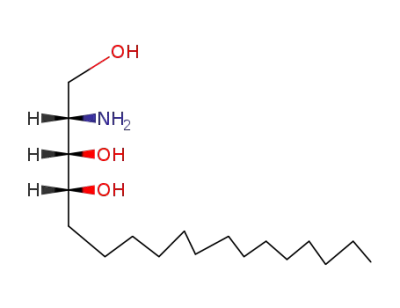
D-ribo-phytosphingosine
| Conditions | Yield |
|---|---|
|
With
lithium aluminium tetrahydride;
In
tetrahydrofuran;
1.) 30 min, RT; 2.) 1 h, reflux;
|
95% |
|
With
hydrogen;
palladium on activated charcoal;
In
methanol;
at 20 ℃;
for 48h;
|
94% |
|
With
pyridine; hydrogen sulfide; water;
Ambient temperature;
|
87% |
|
With
hydrogen sulfide;
In
pyridine; water;
for 168h;
Ambient temperature;
|
87% |
|
With
palladium 10% on activated carbon; hydrogen;
In
methanol;
at 25 ℃;
for 3h;
|
87% |
|
With
triphenylphosphine;
In
pyridine; water;
|
![tert-butyl [(2S,3S,4R)-1,3,4-trihydroxyoctadecan-2-yl]carbamate](/upload/2025/12/1b15e8eb-6bdd-4338-8daa-fd4bd9c55d39.png)
tert-butyl [(2S,3S,4R)-1,3,4-trihydroxyoctadecan-2-yl]carbamate


D-ribo-phytosphingosine
| Conditions | Yield |
|---|---|
|
With
trifluoroacetic acid;
In
water;
at 20 ℃;
for 0.5h;
|
82% |
|
With
trifluoroacetic acid;
In
water;
at 20 ℃;
for 0.5h;
Inert atmosphere;
|
82% |
|
With
trifluoroacetic acid;
at 20 ℃;
for 0.5h;
|
43% |
|
With
hydrogen cation;
|
|
|
With
trifluoroacetic acid;
In
water;
for 0.25h;
Ambient temperature;
|
997 mg |
|
With
trifluoroacetic acid;
In
water;
at 20 ℃;
for 0.333333h;
|
|
|
With
trifluoroacetic acid;
In
dichloromethane;
at 0 - 20 ℃;
for 0.75h;
enantioselective reaction;
|
31 mg |
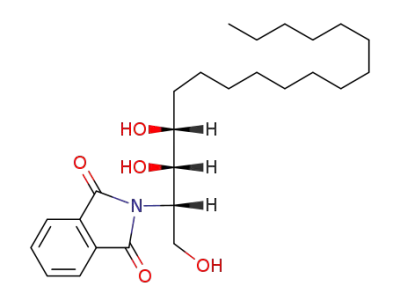
(2S,3S,4R)-2-(phthalimido)-octadecane-1,3,4-triol
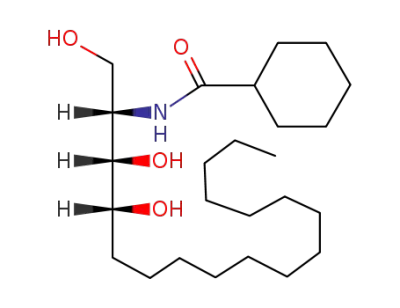
(+)-2-Cyclohexanoylamino-1,3,4-trihydroxy-octadecan
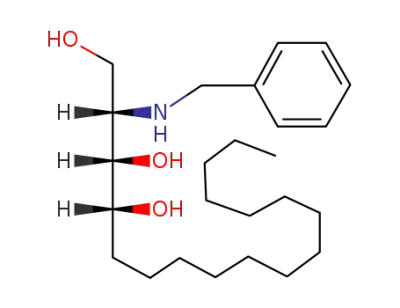
(2S,3S,4R)-2-(benzylamino)octadecane-1,3,4-triol
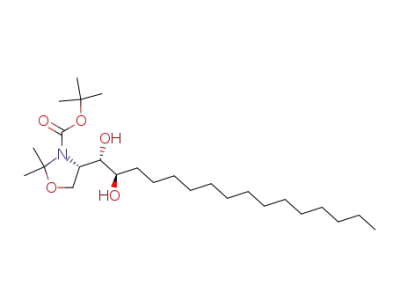
(S)-tert-butyl 4-[(1S,2R)-1,2-dihydroxyhexadecyl]-2,2-dimethyloxazolidine-3-carboxylate
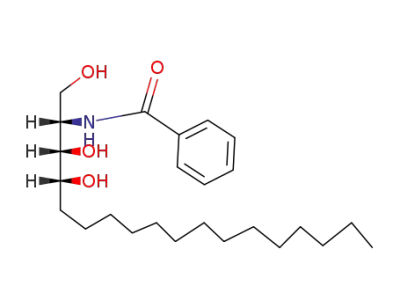
(2S,3R,4R)-2-(N-benzoyl)amino-octadecane-1,3,4-triol
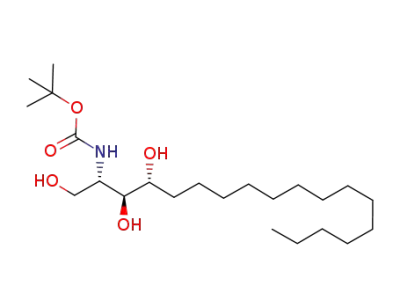
tert-butyl [(2S,3S,4R)-1,3,4-trihydroxyoctadecan-2-yl]carbamate

(2S,3S,4R)-2-azido-1,3,4-octadecanetriol
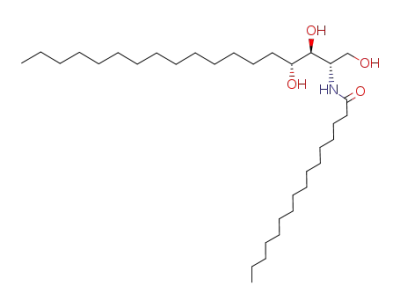
N-palmitoyl-D-ribo-phytosphingosine