Your Location:Home > Products > Amino Acids > L-Lysine Acetate
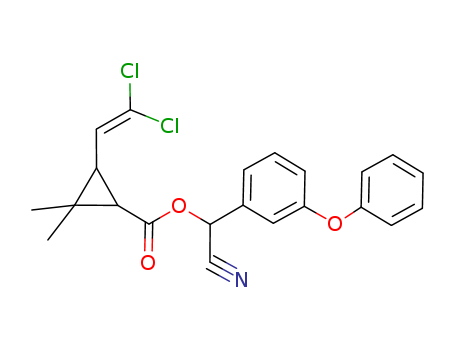


CasNo: 52315-07-8
MF: C22H19Cl2NO3
Appearance: Viscous semisolid
|
Toxicology |
Cypermethrin is a moderately toxic material by dermal absorption or ingestion. Symptoms of high dermal exposure include numbness, tingling, itching, burning sensation, loss of bladder control, incoordination, seizures, and possible death. Pyrethroids like cypermethrin may adversely affect the central nervous system. Symptoms of high-dose ingestion include nausea, prolonged vomiting, stomach pains, and diarrhea which progresses to convulsions, unconsciousness, and coma. Cypermethrin is a slight skin or eye irritant, and may cause allergic skin reactions. The oral LD50 for cypermethrin in rats is 250 mg/kg (in corn oil) or 4123 mg/kg (in water). EPA reports an oral LD50 of 187 to 326 mg/kg in male rats and 150 to 500 mg/kg in female rats. The oral LD50 varies from 367 to 2000 mg/kg in female rats, and from 82 to 779 mg/kg in mice, depending on the ratio of cis/trans- isomers present. This wide variation in toxicity may reflect different mixtures of isomers in the materials tested. The dermal LD50 in rats is 1600 mg/kg and in rabbits is greater than 2000 mg/kg. |
|
Trade name |
AMMO?; AGROTHRIN?; ARDAP?; ARRIVO?; AVICADE?; BARRICADE?; CCN52?; CNN 52?; CYMBUSH? 2E; CYMBUSH? 3E; CYMPERATOR?; CYNOFF?; CYPERCARE?; CYPERSECT?; CYPERKILL?; CYRUX?; DEMON?; DORSAN-C? (+cypermethrin); DYSECT?; FASTAC?; FLECTRON?; FMC? 30980; FMC 45497; FMC? 45806; FOLCORD?; IMPERATOR?; JF 5705 F?; KAFIL? SUPER; KENCIS?; NAGATA?; NRDC 149?; NRDC 160?; NRDC 166?; NURELLE; POLYTRIN?; PERMASECT C?; PP383?; PREVAIL?; RALO 10?; RIPCORD?; ROCYPER?; RYCOPEL?; SHERPA?; SIPERIN?; STOCKADE?; SUPERSECT?; TOPCLIPPARASOL?; USTAAD?; WL 43467?; WRDC149? |
|
Biological Activity |
Type II synthetic pyrethroid insecticide; an extremely potent, cell-permeable inhibitor of calcineurin. |
|
Contact allergens |
Pyrethroids, also called pyrethrinoids, are neurotoxic synthetic compounds used as insecticides, with irritant properties. Cypermethrin and fenvalerate have been reported as causing positive allergic patch tests, but only fenvalerate was relevant in an agricultural worker. |
|
Potential Exposure |
Pyrethroid insecticide used to control pests on cotton, fruit, and vegetable crops. Also used in commercial and residential settings, ships, laboratories, and food-processing plants. A United States Environmental Protection Agency Restricted Use Pesticide (RUP). |
|
Environmental Fate |
Soil. The major soil metabolite was reported to be 3-phenoxybenzoic acid (Hartley and Kidd, 1987).The typical half-life of cypermethrin in the soil is 30 days, although it can range from two to eight weeks (6, 9). Soil microbes rapidly break down cypermethrin.Cypermethrin has an extremely low potential to move in the soil. It is unlikely to contaminate groundwater because it binds tightly to soil particles. Cypermethrin is stable in sunlight.The average half-life of cypermethrin on foliage is 5 days.United States Environmental Protection Agency. (1989). Cypermethrin Pesticide Fact Sheet. Washington, D.C.Knisel, W.G. (Ed.). (1993). Groundwater Loading Effects of Agricultural Management Systems. (Version 2.10). [Online]. Tifton, Georgia: United States Department of AgricultureAgricultural Research Service. [Online]. http://www.arsusda.gov/ rsml/ppdb.html |
|
Metabolic pathway |
In cabbage plants, (1R)-cis- and (1R)-trans-isomers of cypermethrin undergo epimerization to (1S)-isomers, cis=trans isomerization, ester bond cleavage, hydroxylation of the phenoxy group in the alcohol moiety or the geminal methyl group in the acid moiety, hydration of the cyano group to an amido group with subsequent hydrolysis to the carboxylic acid, and the conjugation of the carboxylic acid, and alcohols with sugars. |
|
Shipping |
UN3349 Pyrethroid pesticide, solid toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous material. UN3352 Pyrethroid pesticide, liquid toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous materials. |
|
Degradation |
Zeta-cypermethrin is stable as a solid but it is readily hydrolysed at alkaline pH. Its half-lives at pH values 7 and 9 (25 °C) were 188-635 and 3 days (PM). By analogy with cypermethrin, the major products should be 3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylica cid (2, DCVA), 3-phenoxybenzaldehyde (9, 3PBAl) and a-carbamoyl-3- phenoxybenzyl 3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate (the amide 3); minor products expected are the a-carboxy analogue of 3 (4) and 3-phenoxybenzoic acid (10,3PBA) (see cypermethrin, Schemes la and lb). Photodecomposition would be expected to be similar to that of cypermethrin. In aqueous solution the DTSo was reported to be 20-36 days (PM). |
|
Toxicity evaluation |
Acute oral LD50 for rats: 250-4,150 mg/kg (pure); 7,180 mg/kg (technical grade) |
|
Incompatibilities |
May react violently with strong oxidi- zers, bromine, 90% hydrogen peroxide, phosphorus trichloride, silver powders, or dust. Incompatible with silver compounds. Mixture with some silver compounds forms explosive salts of silver oxalate. |
|
Waste Disposal |
Incineration would be an effective disposal procedure where permitted. If an efficient incinerator is not available, the product should be mixed with large amounts of combustible material and contact with the smoke should be avoided. In accordance with 40 CFR 165, follow recommendations for the disposal of pes- ticides and pesticide containers. |
|
Definition |
ChEBI: A carboxylic ester resulting from the formal condensation between 3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylic acid and the alcoholic hydroxy group of hydroxy(3-phenoxyphenyl)acetonitrile. |
|
Agricultural Uses |
Insecticide: A U.S. EPA restricted Use Pesticide (RUP) used to control a variety of insects on cotton, fruit and vegetable crops. Also used in commercial and residential settings, ships, laboratories and food processing plants. |
InChI:InChI=1/2C22H19Cl2NO3/c2*1-22(2)17(12-19(23)24)20(22)21(26)28-18(13-25)14-7-6-10-16(11-14)27-15-8-4-3-5-9-15/h2*3-12,17-18,20H,1-2H3/t2*17-,18-,20+/m10/s1
The invention discloses a preparation me...
A new quaternary ammonium bromide salt h...
The phenoxybenzyl moiety of conventional...
The present application relates to new a...
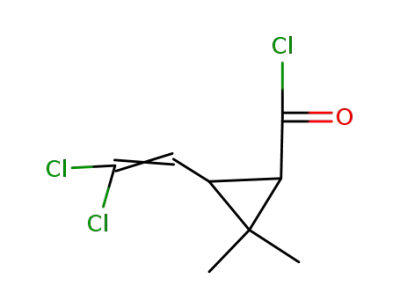
permethric acid chloride

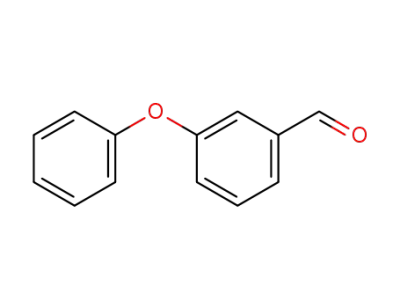
3-Phenoxybenzaldehyde

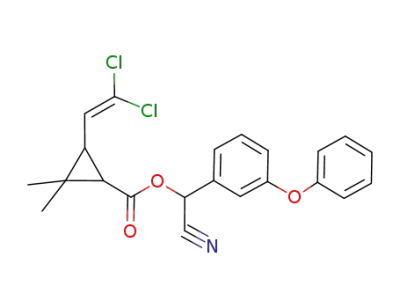
cypermethrine
| Conditions | Yield |
|---|---|
|
In
tetrahydrofuran; dichloromethane; water;
|
95.5% |
|
With
sodium hydroxide;
In
diethylene glycol dimethyl ether; water;
|
90% |
|
With
sodium hydroxide;
In
tetrahydrofuran; water;
|
|
|
In
cyclohexane; water;
|
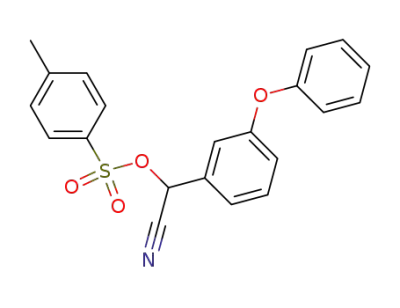
alpha-cyano-3-phenoxybenzyl p-toluene sulphonate

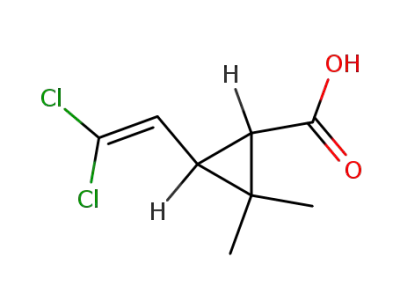
3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylic acid


cypermethrine
| Conditions | Yield |
|---|---|
|
With
tetrabutylammomium bromide; potassium carbonate;
In
water; toluene;
|
99% |
|
With
tetrabutylammomium bromide;
In
water; toluene;
|

permethric acid chloride
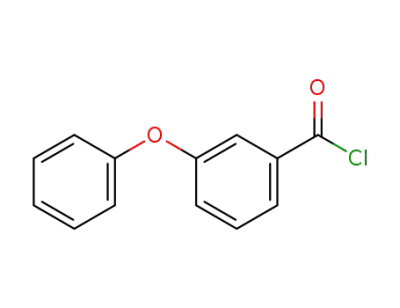
3-phenoxy-benzoyl chloride
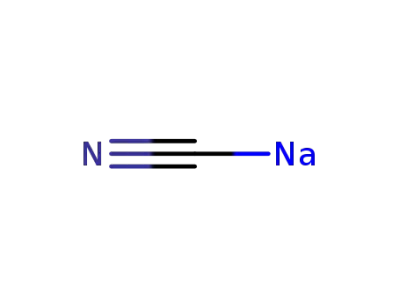
sodium cyanide

3-Phenoxybenzaldehyde
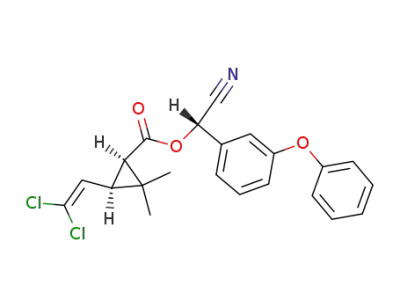
(+)-(S)-α-cyano-3-phenoxybenzyl (1R)-cis-3-(2,2-dichlorovinyl)-2, 2-dimethylcyclopropanecarboxylate
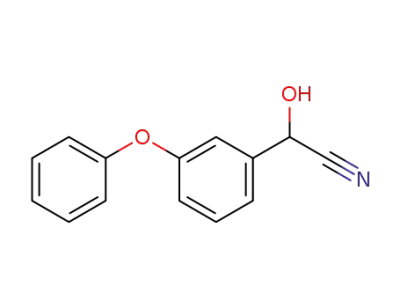
2-hydroxy-2-(3-phenoxyphenyl)acetonitrile

3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylic acid
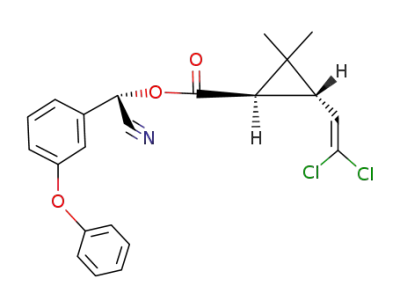
[(S)-cyano-(3-phenoxyphenyl)methyl](1R,3S)-3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropane-1-carboxylate