Your Location:Home > Products > Herbal Extract > 5-HTP
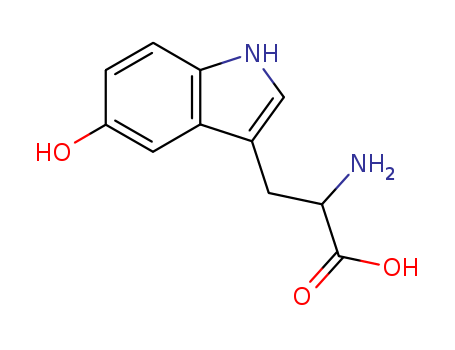


CasNo: 56-69-9
MF: C11H12N2O3
Appearance: White powder
|
Preparation |
The synthesis of 5-hydroxytryptophan (5-HTP) by the condensation of 5-benzyloxygramine with diethyl formaminomalonate, followed by saponification, decarboxylation, and hydrogenolysis was described in 1951 and 1954 and was an application of gramine synthesis, developed by Snyder and Smith ten years before. A few years later, another application of gramine synthesis was reported. In the same year, Frangatos and Chubb reported an application of the convenient tryptophan synthesis developed ten years before by eliminating the difficult and tedious preparation of 5-benzyloxyindole. The p-benzyloxyphenylhydrazone of γ,γ-dicarbethoxy-γ-acetamido-butyraldehyde (I) was prepared and cyclized, without isolation, to form ethyl β-(5-benzyloxyindol-3-)-αcarbethoxy-α-acetamidopropioilate (II). Saponification and partial decarboxylation of II, followed by hydrolysis of the acetamido group, gave 5-benzyloxytryptophan (III). 5-HTP was obtained by hydrogenolysis of III (Figure 1). However, this synthetic method suffers from the difficulty involved in the regioselective hydroxylation of tryptophan.synthesis of 5-hydroxytryptophan (5-HTP) |
|
Manufacturing Process |
Preparation of 5-Hydroxytryptophan: 0.4 gram palladium chloride and 1.7 grams acid-washed charcoal were suspended in 157 ml water and hydrogenated at room temperature and atmospheric pressure until no further hydrogen uptake occurred. A suspension of 14.2 grams 5- benzyloxytryptophan in 175 ml ethyl alcohol was added and the mixture hydrogenated under similar conditions. A hydrogen uptake slightly in excess of theory was obtained. The suspension was warmed for a few minutes on the steam bath and filtered hot. The filter-cake was washed with hot water (3 x 20 ml) and the filtrate evaporated to 20 ml under reduced pressure in a nitrogen atmosphere.The resultant mass of colorless crystals was triturated with 250 ml ice-cold ethyl alcohol under hydrogen, filtered, and washed with cold ethyl alcohol (2 x 15 ml). The 5-hydroxytryptophan (6.9 grams, 69%) had MP (sealed evacuated tube) 288°C, with softening, finally melting at 249° to 247°C (decomposition). Concentration of the liquors under reduced pressure in a nitrogen atmosphere, and trituration as before, gave a second crop (0.9 gram, 9%). The combined crops (7.8 grams) were dissolved in 120 ml hot water, charcoal added, and the mixture filtered hot. The filtrate was concentrated in a nitrogen atmosphere under reduced pressure and ethyl alcohol added. The 5-hydroxytryptophan then crystallized as colorless microneedles (6.5 grams,65%), had MP (sealed evacuated tube) 290°C, with slight softening, finally melting at 295° to 297°C (decomposition). |
|
Therapeutic Function |
Antidepressant, Antiepileptic |
|
Biosynthesis |
5-Hydroxytryptophan, an intermediate molecule in the serotonin biosynthesis pathway, is formed by the addition of a hydroxyl (OH) group to the fifth carbon of the indole ring of tryptophan. It is used as an antiepileptic and antidepressant. |
|
Biochem/physiol Actions |
Immediate precursor of serotonin; L-aromatic amino acid decarboxylase substrate. |
|
Source |
5-HTP is derived from the Griffonia simplicifolia plant. Hypo-allergenic plant fiber is derived from pine cellulose. |
|
Mode of action |
5-Hydroxytryptophan acts primarily by increasing levels of serotonin within the central nervous system. Other neurotransmitters and CNS chemicals, such as melatonin, dopamine, norepinephrine, and beta-endorphin have also been shown to increase following oral administration of 5-HTP. This ability to increase not only serotonin levels in the brain, but also dopamine and norepinephrine, allows 5-HTP to produce some significant and unique effects on brain chemistry and on serotonin-related conditions which other substances, including LT, cannot duplicate. |
|
Definition |
ChEBI: 5-hydroxytryptophan is a tryptophan derivative that is tryptophan substituted by a hydroxy group at position 5. It has a role as a human metabolite and a neurotransmitter. |
|
Application |
5-hydroxytryptophan is a dietary supplement made from the seeds of the African plant Griffonia simplicifolia.5-hydroxytryptophan has been used in alternative medicine as a possibly effective aid in treating depression or fibromyalgia.Other uses not proven with research have included insomnia, alcohol withdrawal, headaches, premenstrual syndrome, binge-eating related to obesity, attention deficit disorder, and muscle spasms in the mouth.5-hydroxytryptophan is often sold as an herbal supplement. There are no regulated manufacturing standards in place for many herbal compounds and some marketed supplements have been found to be contaminated with toxic metals or other drugs. Herbal/health supplements should be purchased from a reliable source to minimize the risk of contamination. |
InChI:InChI=1/C11H12N2O3/c12-9(11(15)16)3-6-5-13-10-2-1-7(14)4-8(6)10/h1-2,4-5,9,13-14H,3,12H2,(H,15,16)/t9-/m0/s1
Several 5-substituted nb-methoxycarbonyl...
5-Hydroxytryptophan (156 mg/l) was ident...
The rate constants for reversible electr...
As a novel compound, an α-alkyl(or aryl)...


5-hydroxytryptophan
| Conditions | Yield |
|---|---|
|
With ethanol; palladium; Hydrogenation;
|
|
|
With sodium hydroxide; palladium; Hydrogenation;
|


5-hydroxytryptophan
| Conditions | Yield |
|---|---|
|
With mannitol; succinic acid; Claviceps sp. PRL 1980; In water; at 25 ℃; for 360h;
|
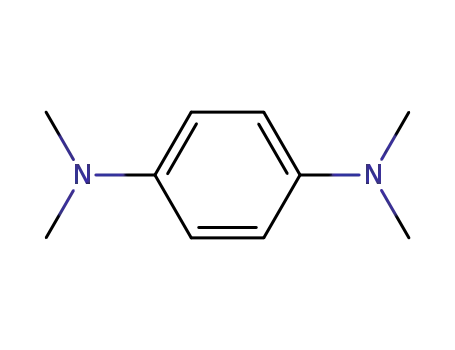
N,N,N',N'-tetramethyl-para-phenylenediamine

5-Hydroxytryptophan radical

4-(N,N-dimethylamino)phenol
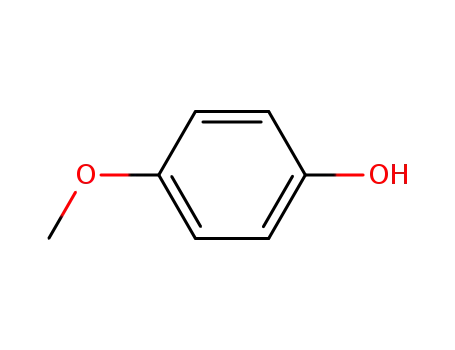
4-methoxy-phenol

5-hydroxytryptophan esthyl ester

N,O-di-Boc-DL-5-hydroxytryptophan dicyclohexylammonium salt
3-(2-aminoethyl)-1H-indol-5-ol

5-Hydroxytryptophan radical