Your Location:Home > Products > Amino Acids > Phenylacetyl-L-Glutamine (PAGLN)


CasNo: 28097-03-2
MF: C30H28 N6 O6 S4
|
Biochem/physiol Actions |
Chaetocin is a competitive inhibitor for S-adenosylmethionine. The specificity of chaetocin for SU(VAR)3-9 makes CHAETOCIN FROM CHAETOMIUM MINUTUM an excellent tool for the study of heterochromatin-mediated gene repression. |
|
Application |
Chaetocin is a natural metabolite isolated from Chaetomium minutum. It is a nonspecific inhbitor of histone lysine methyltransferases which are important epigenetic enzymes . Induces apoptosis in myeloma cell lines and exhibits antiproliferative activity in mammals. Chaetocin from Chaetomium minutum has been used to determine its effects on sensitization of various cells. It has also been used to determine the biological functions of OS-induced heterochromatin formation. |
|
General Description |
Chaetocin is a fungal metabolite with antimicrobial and cytostatic activity. It belongs to the 3,6-epidithio-diketopiperazines class of which gliotoxin, sporidesmin, aranotin, oryzachloride, verticillin A and the melinacidins are members. Chaetocin is a molecular dimer of two five-membered rings cis fused. Interestingly, the chirality of the 3,6-epidithiodioxopiperazine moiety in chaetocin is opposite to the chirality of gliotoxin, sporidesmin, aranotin, and oryzachloride. Unlike these compounds, chaetocin does not have an antiviral activity. This fungal toxin showed strong cytotoxicity against HeLa cells (IC50 = 0.05 mg/ml). |
InChI:InChI=1/C30H28N6O6S4/c1-33-21(39)27-11-25(15-7-3-5-9-17(15)31-19(25)35(27)23(41)29(33,13-37)45-43-27)26-12-28-22(40)34(2)30(14-38,46-44-28)24(42)36(28)20(26)32-18-10-6-4-8-16(18)26/h3-10,19-20,31-32,37-38H,11-14H2,1-2H3
Chaetocin (1), a structurally complex ep...
Chemical Equation Presentation The first...
A highly stereoselective and systematic ...
-
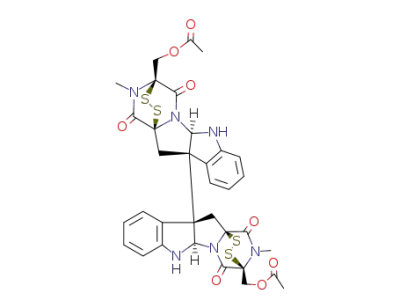
(+)-chaetocin A diacetate

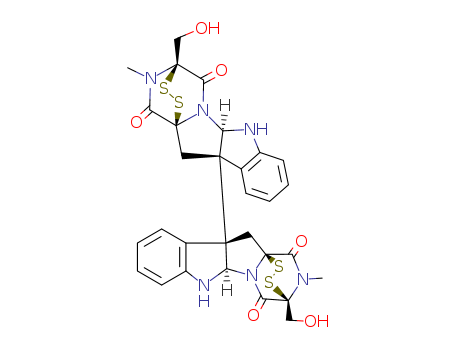
chaetocin
| Conditions | Yield |
|---|---|
|
With
methanol; Otera's catalyst;
In
toluene;
at 85 ℃;
for 7.33333h;
Inert atmosphere;
|
92% |
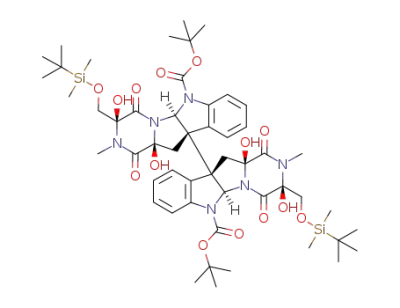
C52H76N6O14Si2


chaetocin
| Conditions | Yield |
|---|---|
|
C52H76N6O14Si2;
With
boron trifluoride diethyl etherate; hydrogen sulfide;
In
dichloromethane;
at -78 - 20 ℃;
Sealed tube;
With
iodine;
In
ethyl acetate;
at 20 ℃;
for 0.0166667h;
|
44% |
|
Multi-step reaction with 2 steps
1: hydrogen sulfide; boron trifluoride diethyl etherate / dichloromethane / -78 - -20 °C
2: iodine / ethyl acetate
With
boron trifluoride diethyl etherate; hydrogen sulfide; iodine;
In
dichloromethane; ethyl acetate;
|
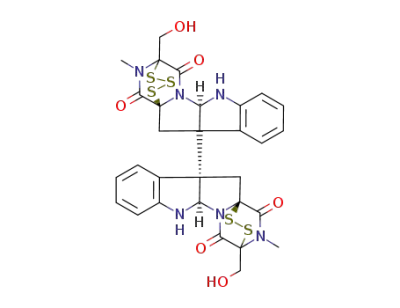
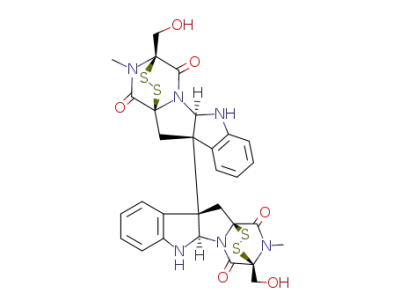
chaetocin B
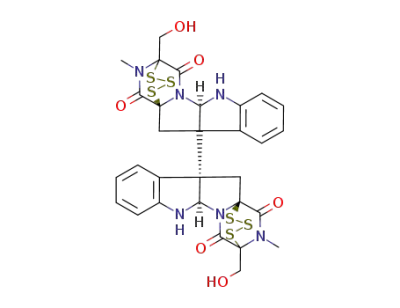
chaetocin C

C52H76N6O14Si2
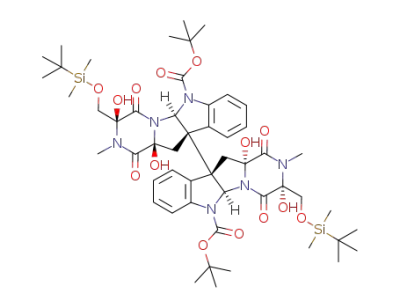
C52H76N6O14Si2