Your Location:Home > Products > Amino Acids > D-Ribose
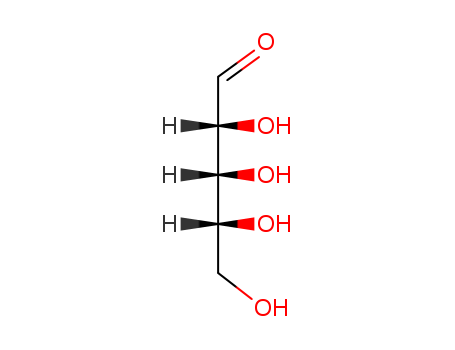


CasNo: 50-69-1
MF: C5H10O5
Appearance: white powder
|
Identification and Nomenclature |
It is identified as a D-ribose and an aldehydo-ribose, being the enantiomer of an aldehydo-L-ribose. Its systematic name is (2R,3R,4R)-2,3,4,5-tetrahydroxypentanal. |
|
Versatility |
Known by various names including ribose, aldehydo-d-ribo-pentose, and ribo-2,3,4,5-tetrahydroxyvaleraldehyde. Its unique structure and properties make it useful in pharmaceuticals, biochemistry, and organic synthesis. |
|
Precursor to D-Ribose |
Serves as a precursor to D-ribose, which is a crucial component of ribonucleic acid (RNA) and certain coenzymes. |
|
Cyclic Forms |
Like most sugars, ribose exists as a mixture of cyclic forms in equilibrium with its linear form, and these readily interconvert especially in aqueous solution. In its linear form, ribose can be recognised as the pentose sugar with all of its hydroxyl functional groups on the same side in its Fischer projection. d-Ribose has these hydroxyl groups on the right hand side and is associated with the systematic name (2R,3R,4R)-2,3,4,5-tetrahydroxypentanal, whilst l-ribose has its hydroxyl groups appear on the left hand side in a Fischer projection. |
|
Preparation |
D-Ribose is obtained by using D-glucose as a raw material, inserting bacteria or Bacillus subtilis for fermentation, and separating and refining the fermentation product.(By fermentation technology) |
|
Definition |
ChEBI: D-ribose is a ribofuranose having D-configuration. It is a naturally occurring monosaccharide found in the cells and particularly in the mitochondria is essential in energy production. |
|
Biological Activity |
D-ribose is a natural sugar that our bodies produce for a variety of purposes most notably encrgy in the form of ATP(adenosine triphosphate), which powers every cell in our body. Ribose is crucial for the synthesis of energy (ATP) and without it, your cells run out of energy and the health of the cell is compromised. Supplementing with ribose improves cell function by restoring energy. One of the most vital organs to suffer energy depletion is the heart. That is why so much of the research on D-ribose has focused on the impact of supplement ribose in heart disease. Although ribose is classified as a sugar, when taken orally, it does not have any impact on blood sugar like glucose. Instead, it is used for energy production and energy recovery. Every cell in the body has the capacity to make ribose. But more ribose may be needed than the cell can produce when cells are metabolically stressed, such as with strenuous exercise or disease. When the cells are metabolically stressed they tend to consume more glucose rather than producing more ribose. This can dramatically affect recovery. |
|
Biochem/physiol Actions |
Ribose is an aldopentose monosaccharide that is phosphorylated into D-ribose 5-phosphate by ribokinase. |
|
Side effects |
D-ribose is a dietary supplement approved by the US FDA. It is generally considered safe for dosage of ribose is less than 15g/d. However, possible side effects include diarrhea, stomach discomfort, nausea, headache, and low blood sugar. |
|
Purification Methods |
Crystallise -D(-)-ribose from aqueous 80% EtOH, dry it under vacuum at 60o over P2O5 and store it in a vacuum desiccator. It exhibits a complex mutarotation with : [] D 10 -23.1o (1.5minutes), -21.3o (5minutes), -19.5o (10minutes), -19.1o (30minutes), -21.2o (60minutes), -23.1o (120minutes), -23.7o (300minutes), (c 4.5, H2O) [Phelps et al. J Am Chem Soc 56 748 1934]. 1H NMR in D2O at 44o shows 17% -pyranose, 59% -pyranose, 9% -furanose and 15% -furanose forms with furanose -H at 5.34ppm (J 3.0Hz) and -H at 5.31 (J 1.7Hz) [Angyal Adv Carbohydr Chem 42 15 1984, Angyal & Pickles Aust J Chem 25 1711 1972]. The phenylhydrazone crystallises from aqueous pyridine in yellow needles, m 163-164o, and the benzylphenylhydrazone has m 127-128o [Snowden J Am Chem Soc 72 808 1950.] [Beilstein 1 IV 4211.] |
InChI:InChI=1/C5H10O5/c6-1-2-3(7)4(8)5(9)10-2/h2-9H,1H2/t2-,3-,4-,5-/m1/s1
Understanding ribose reactivity is a cru...
The problem of β-nucleoside formation un...
Two new bisamide compounds, eximiamide A...
This work describes the application of t...
Six new isochroman derivatives (annulohy...
-
-
-
Two new compounds (1 and 2), together wi...
Genomic sequence analysis of Acinetobact...
Four new rarely occurred seco-dammarane ...
The present disclosure is related to ele...
Marine organisms are a valuable source o...
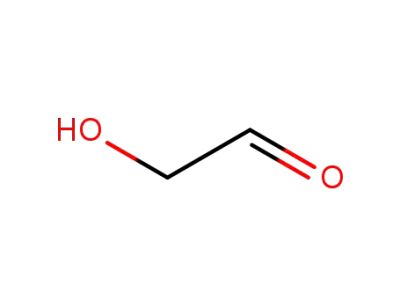
Glycolaldehyde

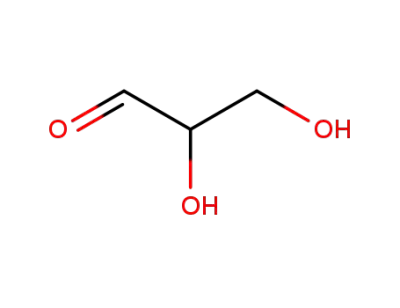
Glyceraldehyde

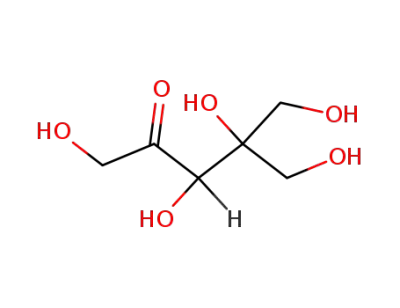
DL-dendroketose

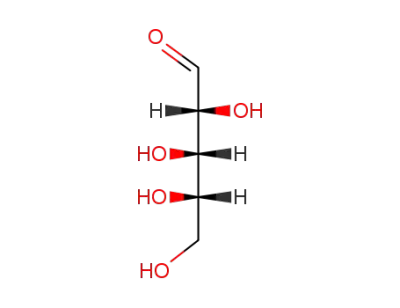
arabinose

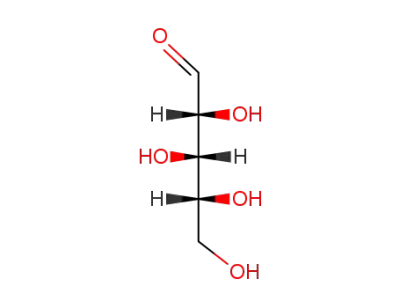
(+/-)-xylose

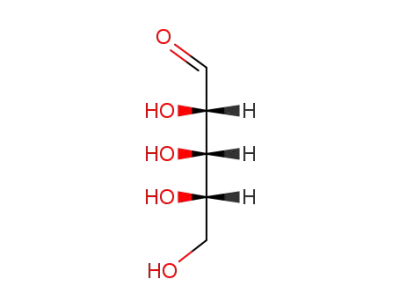
rac-Ribose

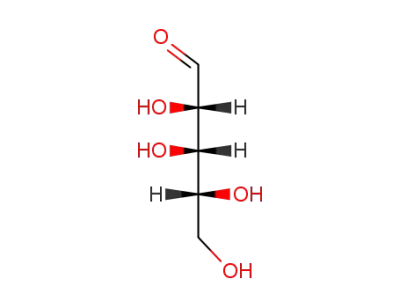
rac-lyxose

Dendroaldose
| Conditions | Yield |
|---|---|
|
With
calcium hydroxide;
at 23 ℃;
Mechanism;
Kinetics;
|

Glycolaldehyde


Glyceraldehyde


DL-dendroketose


arabinose


(+/-)-xylose


rac-Ribose


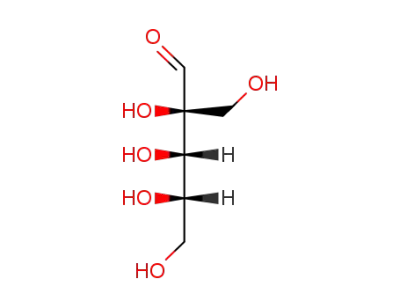
rac-lyxose


Dendroaldose
| Conditions | Yield |
|---|---|
|
With
calcium hydroxide;
at 23 ℃;
Mechanism;
Kinetics;
|
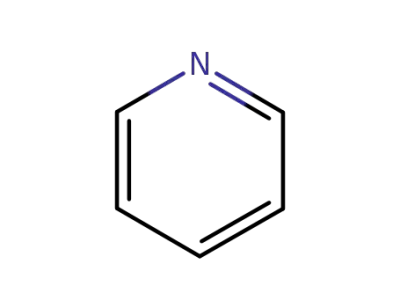
pyridine
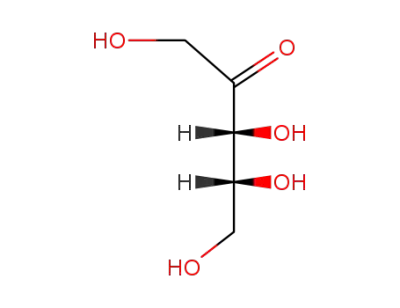
D-ribulose
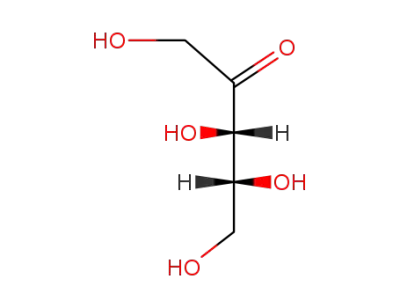
xylulose
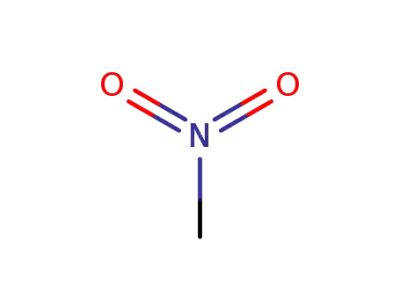
nitromethane

D-ribulose
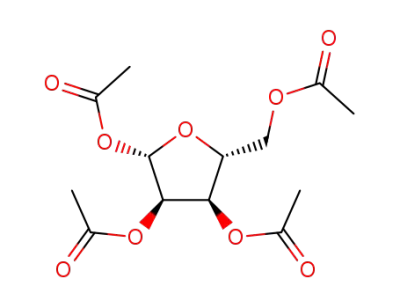
1,2,3,5-tetraacetylribose
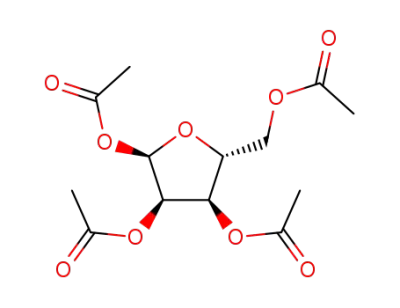
tetra-O-acetyl-D-ribofuranose
1,2,3,4-tri-O-acetyl-α-D-ribopyranose